Next: Modelling of the emissions Up: Aurora and artificial airglow Previous: Aurora and artificial airglow Contents
Subsections
(F)our Auroral lines
5577 Å - The auroral green line
The emission of the ``auroral green line'' at 5577 Å is in
general the brightest emission,
giving the aurora its characteristic green
colour. It is emitted by atomic oxygen in its transition from the
second lowest excited electronic state ![]() to the lowest
excited electronic state
to the lowest
excited electronic state ![]() . The three main sources are
energy transfer from
. The three main sources are
energy transfer from
![]() , dissociative recombination
and direct electron excitation. Energy
transfer via the reaction:
, dissociative recombination
and direct electron excitation. Energy
transfer via the reaction:
has a yield of approximately 0.4 in aurora ( Strickland et al., 2000). According to Hill et al. (2000) the reaction rate depends on the neutral temperature and the vibrational level of the excited nitrogen molecule. The dissociative recombination of
where
The yield of
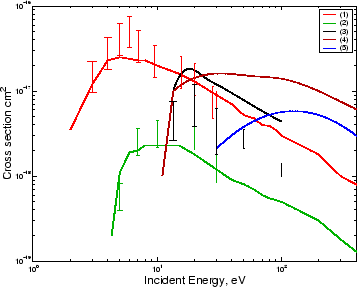
With the cross section ( Itikawa and Ichimura, 1990) shown in green in Figure 2.1, this process is a source of the
 |
6300Å - The auroral red line
The 6300 Å emission, the ``red line'', is sometimes seen in the
upper border of auroral arcs. It is emitted by atomic oxygen in its
transition from the lowest excited electronic state ![]() to the
atomic ground state
to the
atomic ground state ![]() . The metastable
. The metastable ![]() state has a
radiative lifetime of 107 s. The main sources of
state has a
radiative lifetime of 107 s. The main sources of ![]() are direct
electron impact excitation of atomic oxygen:
are direct
electron impact excitation of atomic oxygen:
with the excitation cross section as shown in Figure 2.1 and dissociative recombination of
with an
Due to the 107 s radiative lifetime of ![]() , the emission of the 6300
Å line competes with collisional de-excitation, quenching, as a
cause of energy loss of
, the emission of the 6300
Å line competes with collisional de-excitation, quenching, as a
cause of energy loss of
![]() , where the excitation energy is lost as kinetic, vibrational
or rotational energy of the colliding particles without emission of
photons. Here collisions with molecular oxygen and nitrogen follow:
, where the excitation energy is lost as kinetic, vibrational
or rotational energy of the colliding particles without emission of
photons. Here collisions with molecular oxygen and nitrogen follow:
| (2.7) | ||
| (2.8) |
with the rate coefficients given by ( Mantas and Carlson, 1996):
| (2.9) | ||
| (2.10) |
and with atomic oxygen:
with the rate coefficient ( Solomon et al., 1988) given by:
have to be taken into account. Further,
with a quenching rate ( Rees, 1989):
This implies that the emission of the 6300 Å mainly originates at high altitudes with peak volume emission rates typically between 200 and 400 km. At lower altitudes
8446 Å
The 8446 Å emission, from atomic oxygen in the near
infrared, results from the transition
![]() . These states have a slightly higher excitation energy than
the upper states of the auroral green and red lines:
. These states have a slightly higher excitation energy than
the upper states of the auroral green and red lines:
![]() has an excitation energy of 10.99 eV. The upper
state
has an excitation energy of 10.99 eV. The upper
state ![]() is excited mainly by direct electron
impact on
is excited mainly by direct electron
impact on ![]() :
:
but a minor source is dissociative electron excitation:
The
4278 Å -- a
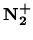 1NG line
1NG line
The ![]() emission 4278 Å in the blue region of the visible
spectum is emitted by the transition from the
emission 4278 Å in the blue region of the visible
spectum is emitted by the transition from the
![]() state to the first vibrational level of electronic ground state of the
molecular nitrogen ion
state to the first vibrational level of electronic ground state of the
molecular nitrogen ion
![]() . According to
Borst and Zipf (1970), 11 % of the
. According to
Borst and Zipf (1970), 11 % of the ![]() ionization is to the
ionization is to the
![]() state of which 19 % emits 4278 Å photons. As a
consequence a total of 2.13 % of the
state of which 19 % emits 4278 Å photons. As a
consequence a total of 2.13 % of the ![]() ionization results in
emission of 4278 Å ( Vallance Jones, 1974). Later emission cross
sections have been reported ( Itikawa et al., 1986) and are shown in
Figure 2.1.
ionization results in
emission of 4278 Å ( Vallance Jones, 1974). Later emission cross
sections have been reported ( Itikawa et al., 1986) and are shown in
Figure 2.1.
Next: Modelling of the emissions Up: Aurora and artificial airglow Previous: Aurora and artificial airglow Contents
copyright Björn Gustavsson 2000-10-24